Ionic Bonds Could Be Best Described as
A bond in which electrons are shared equally. Therefore by definition ionic bonds are formed between anions and cations.

Ionic Bond Electrovalent Bond Definition Properties Electronegativity Examples With Videos
In fact it is the strongest type of chemical bond that exists.
. D the sharing of electrons. An allele is the part of a gene. Have low boiling points C.
Which chemical species can easily form an ionic bond with a cation. Covalent because valence electrons are shared B. Covalent because valence electrons are transferred.
An ionic bond forms between atoms of A. An electrostatic attraction between oppositely charged ions. It is a measure of the strength of.
14Which compound contains ionic bonds. Types of chemical bonds including covalent ionic and hydrogen bonds and London dispersion. Based upon their electronegativities the bond could be described as.
1 Get Other questions on the subject. H and Cl 8. Ionic compounds containing hydrogen ions H are classified as acids and those containing hydroxide OH or oxide O 2 ions are classified as bases.
Ionic bonds could be best described as a. SYI1 EU SYI1B LO SYI1B1 EK Chemical bonds hold molecules together and create temporary connections that are essential to life. 1 An ionic bond is best described as A the sharing of electrons.
An electrostatic attraction between oppositely changed ions d. Which term best describes the shape of the I 3-ion. A bond formed when 2 atoms share electrons b.
Ionic bonding also commonly called electrovalent bonding is a type of chemical bonding that involves the bonding formed by the electrostatic attraction between atoms with a high electronegative difference as seen in group 1 atoms and group 7 atoms. An ionic bond is a chemical bond between two atoms in which one atom seems to donate its electron to another atom. Elimination of feces and some metabolic wastes.
Ionic bonding is a form of chemical connection in which one atom loses valence electrons and gains them from another. Which of the following compounds contains a bond with the greatest degrees of ionic character. D the attraction between two nonmetal atoms.
Acovalent because valence electrons are shared Bcovalent because valence electrons are transferred Cionic because valence electrons are shared Dionic because valence electrons are transferred 15The bonds in BaO are best described as Aan ionic solid Ba network solid Ca metallic solid Da molecular solid. The attractive electrostatic interactions between two ions of opposite charge form an ionic bond. E the attraction between 2 metal atoms.
Which of these best describes an ionic bond. Ionic bonds form between two atoms that have different electronegativity values. The table below gives a formula and the carbon-nitrogen bond.
A P b Ca c Al d O e Se. Which of the following would form compounds with fluorine in which the bond is the least ionic. For both atoms involved this exchange results in a more stable noble gas electrical state.
Have high melting points B. An electrostatic attraction between anions. P and Cl D.
Group 1 atoms are known as alkali metals and they have 1 valence electrons in their outermost shell. A force that holds two oppositely charged ions together. Ions can be atomic or molecular in nature.
Compounds by contrast can be separated into their constituent atoms only by chemical means breaking bonds. Biology 21062019 2200 misscheoneyo. PO43-Which statement is true about crystal lattice energy.
An allele is a variation of a gene that can be expressed as a phenotype. Ionic compounds typically have high melting and boiling points and are hard and brittle. Movement of food particles through the wall of the alimentary canal.
Which of the following compounds is most likely to be on the borderline between ionic and covalent compounds. Ionic bonds could be best described as. An ionic bond is best described as A the attraction between 2 nonmetal atoms B the transfer of electrons from one atom to another.
The atom that loses the electrons becomes a positively charged ion cation while the one that gains them. The covalent bond is also. A type of bond important in tying different parts of the same molecule together into a three-dimensional structure.
A chemical bond involving carbon and hydrogen C-H is present in many biological molecules. The properties of atoms and molecules are not changed when they become part of a mixture. The ionic bond is the electrostatic force of attraction between two oppositely charged ions.
CO 3 2- is a molecular anion. Required to break a chemical bond. Covalent bonds on the other hand appear to involve two atoms sharing electrons reach a more stable electron configurationSome compounds contain both ionic and covalent bondsThese compounds contain polyatomic ionsMany of these.
An electrostatic attraction between anions. A bond formed when 2 atoms share electrons. A bond formed when 2 atoms share electrons.
A characteristic of ionic solids is that they A. Recognizing Compounds With Ionic Bonds. The bonds in BaO are best described as A.
2 Depending on the mixture its components can be separated by physical means straining filtering evaporation and so on. All other ionic compounds without these ions are known as salts. Which statement best describes the relationship between an allele and a gene.
A firm handshake c. When drawing out Lewis structures how many electrons surround the most stable. A bond in which electrons are shared unequally.
C the attraction that holds the atoms together in a polyatomic ion. Electrostatic attraction between ions. Ionic bonds could be best described as.
Such a bond forms when the valence outermost electrons of one atom are transferred permanently to another atom. The definition of the chemical bond as a shared electron pair could be extended to describe the dative bond and the elaboration of Lewis acidbase interactions. B the transfer of electrons from one atom to another.
I and Cl B. Reactions that break chemical bonds of food particles. K and Cl C.
Which best describes how an ionic bond forms. Chemistry questions and answers. An electrostatic attraction between oppositely charged ions.
A bond in which electrons are completely lost or gained by the atoms involved. Ionic compounds tend to be solid in nature and they usually have very high melting points as the ionic bonds are quite strong. Ionic bonds join metals to non-metals.
Because the ability to attract electrons is so different between the atoms its like one atom donates its electron to the other atom in the chemical bond. Ionic bond also called electrovalent bond type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Absorption is best described as the.
You can recognize ionic compounds because they consist of a metal bonded to a nonmetal. C the attraction that holds the atoms together in a polyatomic ion.

Ionic Bonding Biology Definition Role Expii
Ionic Bonding Biology Definition Role Expii

Ionic Bonds Vs Covalent Bonds Chemtalk
Ionic Bond Examples Biology Dictionary
Ionic Bond Examples Biology Dictionary

The Ionic Bond Boundless Chemistry
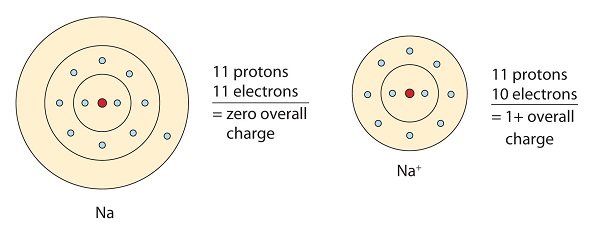
1 3 Ionic And Covalent Bonds Chemistry Libretexts

Ionic Bond Definition Properties Examples Facts Britannica
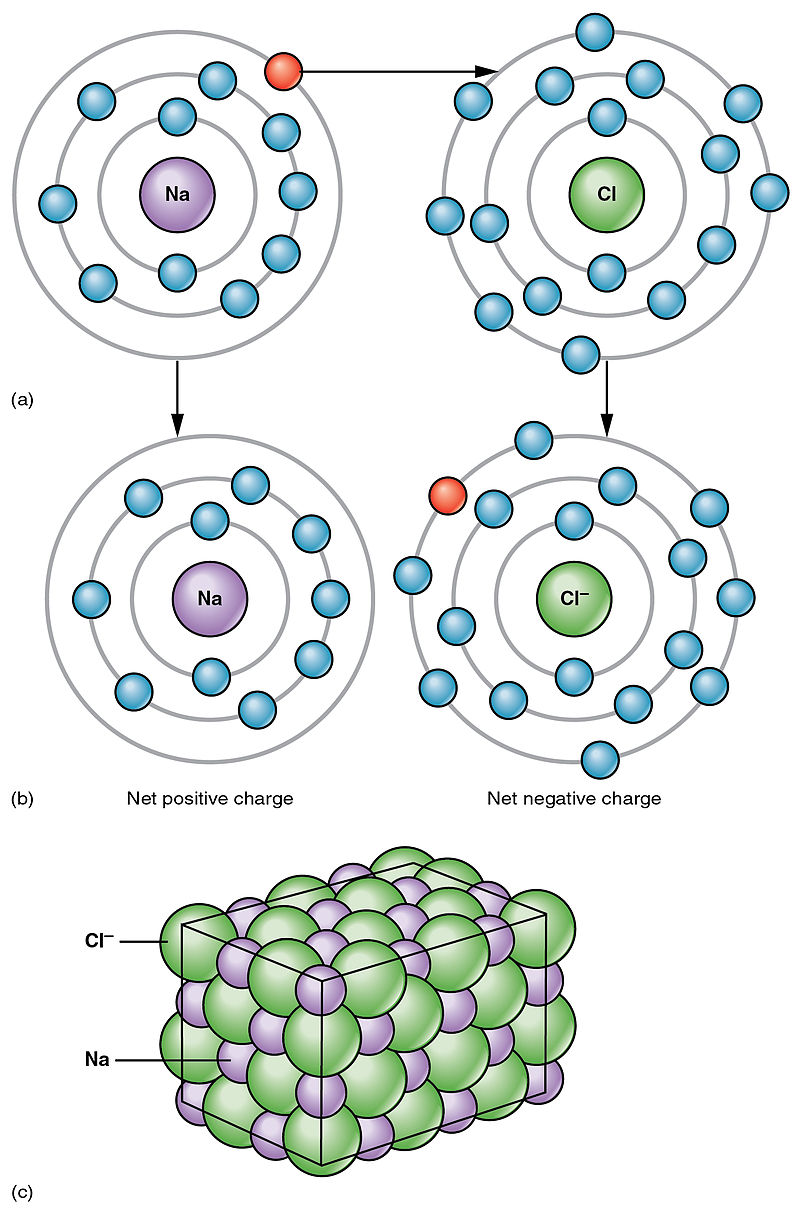
Difference Between Covalent And Ionic Bonds
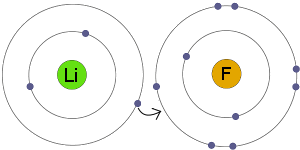
Definition Of Ionic Bonding Chemistry Dictionary

Ionic Bonding Biology Definition Role Expii

Difference Between Ionic And Covalent Bonds Compare The Difference Between Similar Terms
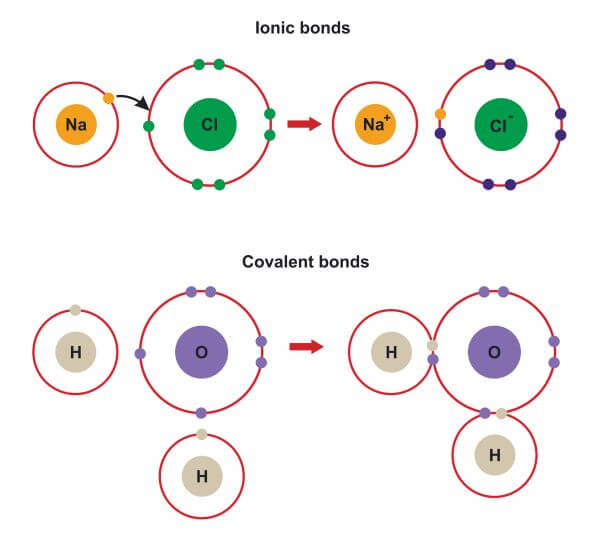
Ionic Bond Examples Biology Dictionary

Ionic Bond Facts Definition Properties Examples Diagrams

What Are Ionic Compounds Definition Structure Properties Examples With Videos Of Ionic Compounds Ionic Character

Covalent Vs Ionic Bond Definition 11 Key Differences Examples The Chemistry Notes
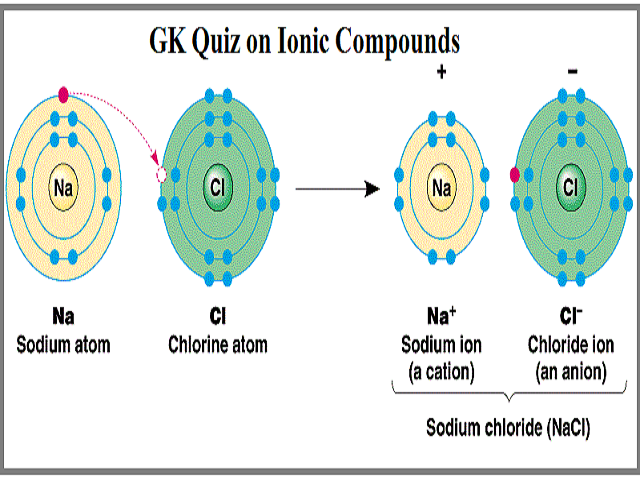

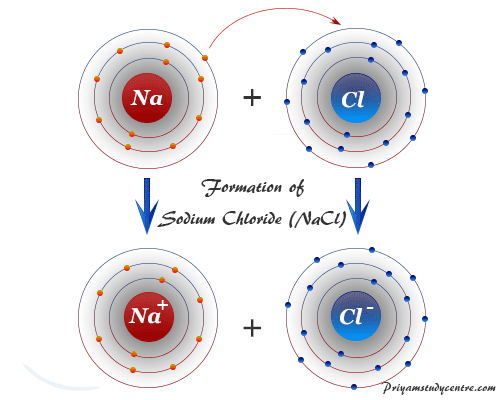
Comments
Post a Comment